LP shunt vs. VP shunt
The Lumboperitoneal Shunt as an Alternative
Foreword
Since the emergence of hydrocephalus shunt therapy in the 1950s, cerebrospinal fluid diversion from the cerebral ventricles to the abdominal cavity (VP shunt/VPS) has dominated with an application frequency of over 70%. In contrast, the alternative drainage from the lumbar region (LP shunt/LPS) was suspected to be more prone to complications. However, in the last two decades, the use of LP shunting has increased significantly.
show more
History of Shunt Therapy
Since the 1950s, the implantation of a shunt system has been the standard therapy for the treatment of hydrocephalus. The shunt consists of a tube-valve system that drains excess cerebrospinal fluid (CSF) into another body cavity where it can be reabsorbed. At the same time, there have been and continue to be attempts to develop alternative treatments, for example, by means of drugs or by special surgical interventions, such as endoscopic third ventriculostomy (ETV) or partial or complete removal of the choroid plexus, the structures in the brain where cerebrospinal fluid is formed (1-5). However, these have ultimately remained without major relevance to date and have not been able to replace shunt therapy.
Implantation of a shunt is generally neither dangerous nor difficult compared to other neurosurgical procedures. A typical drainage system (see below) consists of at least silicone catheters and a valve that regulates intracranial pressure. The catheters, valve, and other optional components are implanted subcutaneously, meaning under the skin. The catheters are passed under the skin with a tunneling instrument, and the valve is fixed in a skin pocket using a small incision. Only the tips (openings) of the inflow and outflow catheters invasively penetrate the respective body cavities, depending on the type of shunt.
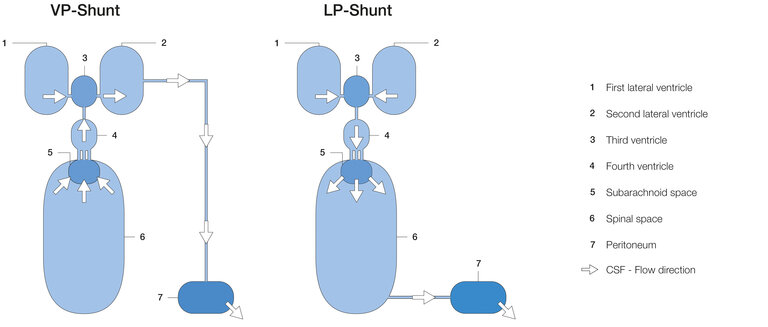
Types of shunt derivation: VP, LP, VA
Theoretically, a large number of body cavities can be considered for derivation. However, only three of these drainage options have any practical significance. All other types of drainage are used only in rare special cases.
1. ventriculoperitoneal (VP): from the cerebral ventricles (head) into the peritoneum (abdominal cavity)
2. lumboperitoneal (LP): out of the spinal canal into the peritoneum.
3. ventriculoatrial (VA): from the brain ventricles (head) into the right atrium of the heart.
These three types of derivation have been used since the 1960s and are well established operations. As shown in Figure 1, the valves and other optional components are placed retroauricularly (i.e., behind the ear), in the thoracic or in the lumbar region, among others.

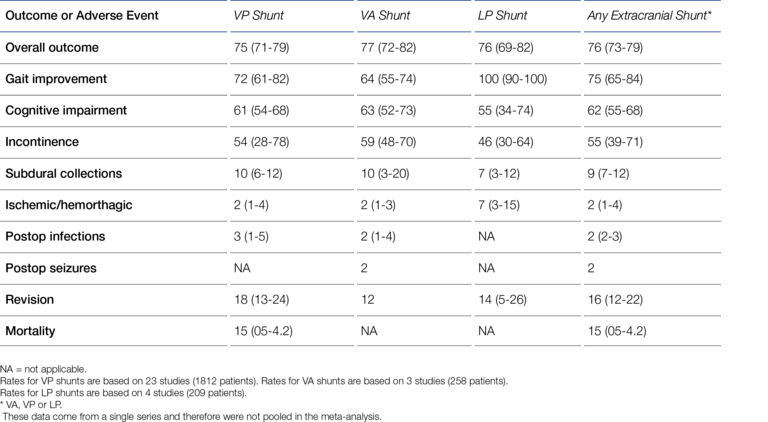
To place the ventricular catheter, a burr hole must be made through the cranial dome (skull bone) to provide access to the ventricular system. Despite this obvious invasiveness, the VP shunt established itself against the LP and VA shunt early on, thus becoming the standard method. According to Aschoff, in 1999, 98% of pediatric shunt procedures and more than 80% of adult shunt procedures were VP shunt procedures (6). Giordan et al. recently (2018) published a systematic meta-analysis of 2,461 iNPH (Idiopathic Normal Pressure Hydrocephalus) shunt patients* that shows the same trend as Aschoff's data (7):
- VP-shunt: 1812 patients (74%)
- LP-shunt: 209 patients (8.5%)
- VA-shunt: 258 patients (10.5%)
- ETV: 182 patients (7.4%) (ETV = Endoscopic Third-Ventriculostomy).
Traditionally, LP drainage was used whenever access to the ventricles was difficult, i.e., especially in patients with narrow ventricles or slit ventricles. The proximal catheters - predominantly ventricular catheters (VC) and lumbar catheters (LC) - are often closed catheters whose tips contain small drainage holes through which CSF can enter. Equally, however, catheters that are open at the end are also common.There is strong evidence that misplacement of the ventricular catheter significantly increases the complication rate for the shunt, particularly because the CSF entry holes are blocked by excessively narrow brain tissue (8-10). Therefore, the LP shunt plays a particularly important role in the care of IIH patients (IIH = Idiopathic Intracranial Hypertension, formerly PTC = Pseudo-Tumor Cerebri or BIH, Benign Intracranial Hypertension) who have narrow ventricles (11). Azad et al. recently (2020) published a typical example in which 32% of 1,082 IIH patients received an LP shunt (12).
However, LP diversion is indicated exclusively in "hydrocephalus communicans" (communicating hydrocephalus), i.e., hydrocephalus forms that do not result from blockage of a CSF flow path (13). For example, if the aqueduct between the third and fourth ventricles or spinal space is blocked, LP drainage would not only be therapeutically ineffective but even contraindicated, because the resulting pressure gradient between the upper cerebral hemisphere and the spinal space would promote dangerous displacement and entrapment of brain parts ("tonsilar herniation") (14-16). This pressure gradient arises because the ventricles are located in the upper part of the brain and a major part of CSF production takes place there.
Advantages of the LP shunt
At first glance, the LP shunt has some advantages. Although rarely practiced, this derivation option can be performed under local anesthesia if it offers advantages for the patient. Another particularly appealing aspect is that, in contrast to the VP shunt, the skull bone does not have to be pierced and therefore there is no risk of brain injury or even brain hemorrhage during shunt placement (17). Overall, LP shunt placement can be considered less invasive compared with VP shunt placement. However, for quite some time, LP shunting was generally "suspected" to be associated with unacceptably high complication rates compared with VP shunts (18-20). Larger, also randomized studies and meta-reviews presented in the last decade have been able to dispel this suspicion though (21-23). A good recent example is a meta-review by Giordan et al. who, on the basis of extensive data from more than 2,000 patients, demonstrated (at least) the equivalence of LP and VP shunts in terms of safety and performance, i.e., complication rates and therapeutic success ("outcome") (cf. Figure 2) (7).
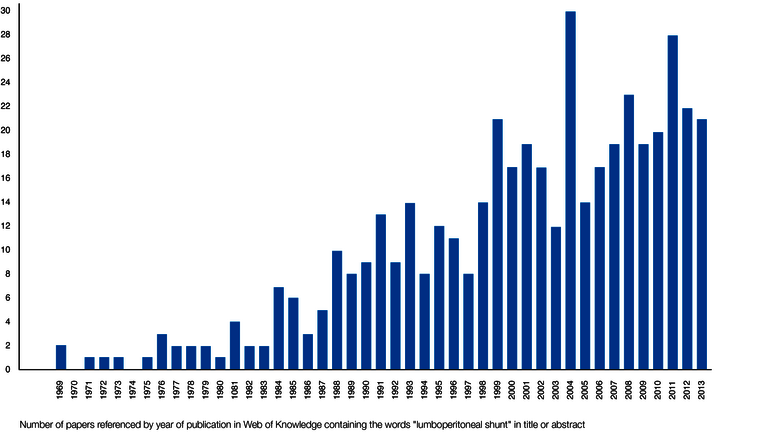
Such positive findings have led to an increase in the rate of LP shunts, especially in the Asian region such as Japan, but also worldwide. Already in 2011, more iNPH patients in Japan were treated with LP shunts than with VP shunts (21,24). Figure 3 reflects the increasing use of LP shunts worldwide through a literature search for the keyword "lumboperitoneal shunt" in the interdisciplinary "Web of science" (25).
Specific complications of the LP shunt
The possible complications and their causes overlap only partially between VP and LP shunts. In addition to commonalities, such as mechanical valve problems, each shunt type is also associated with specific complications. Jusue-Torres et al. analyzed the different LP complication causes from 602 LP shunt studies between 1971 and 2013 based on 2,871 treated patients (25). The result (reduced to the main factors) is shown in the following Figure 4. The percentages refer to the cumulative total number of all complications.
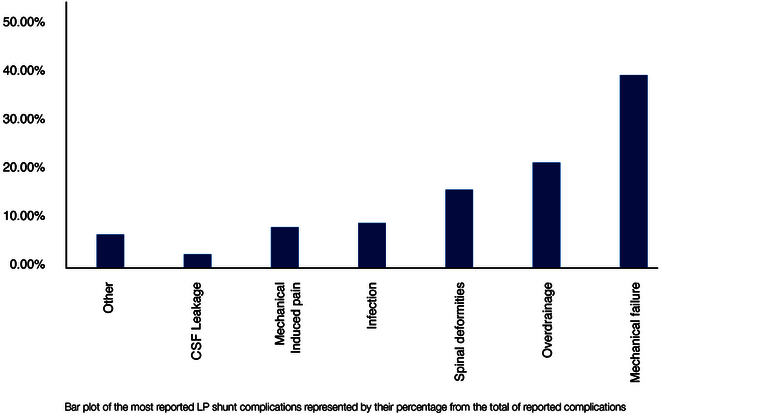
Because of the implantation in the particularly mobile and often moved lumbar region of the body, mechanical complications dominate in LP shunts. These include catheter breakage, valve rupture, catheter migration, and misplacement, as well as obstruction, which is also common in VP shunts (26). In agreement with other studies, overdrainage is also one of the most frequent complications (also as in VP shunts) (25,27,28).
In contrast, the following complications are specific to the LP shunt and are very rare or even excluded in a VP shunt (see Fig. 4):
- "Spinal Deformities (16%)."
- "Mechanical-Induced Pain (8%)."
- "CSF leakage (3%)" and
- "Others (5.5%)".
The first group, "spinal deformities," essentially includes growth disorders of the spine that can be triggered by an LP shunt, insofar as it was implanted at a very young age, for example in children. The second group includes various back pains (e.g., "radiculopathy") that may result from irritation of spinal nerves. The LP shunt must be revised in such cases. The third group, CSF leaks, may result from leakage of the spinal dura (the "hard meninges" that also surrounds the spinal canal), since the Tuohy cannula used for lumbar puncture must have a slightly larger diameter than the LP catheter ultimately placed. The group "Others" is dominated with 5.5% by the already mentioned "Tonsilar Herniation", i.e. slippage and entrapment of brain parts, which was observed exclusively during LP drainage. The reason for this lies in hydrostatic and hydrodynamic processes, which will be discussed below.

At this point, a frequent and relevant misunderstanding must be addressed, which probably stems from inaccurate formulations in the literature. In addition to the problem of suboptimal valve setting, it is well known that in common parlance over- and underdrainage is mainly caused by the so-called "siphon effect in the standing position". In the upright position, the ventricles are 30-60cm above the drainage site, so that the cerebrospinal fluid column hanging in the catheter generates a strong suction in the ventricles due to its own weight pulling downwards ("gravity"). In this regard, for example, Ho et al. 2023 write (22):
"Although rare, over drainage causing slit ventricle syndrome, intracranial hypotension syndrome, chronic subdural effusion, or subdural hemorrhage may also be the disadvantages of VPS.
On the other hand, the lumbar exit and peritoneal entry for LPS are generally at the same level when the patients are upright to minimize the effect of gravity, and the siphoning effect is negligible."
In the same vein, Miyake writes (26):
"All valves except valves with a membrane-type antisiphon mechanism can be implanted in any location in LP shunts.
Use of valves with membrane type antisiphon mechanisms is unnecessary because the siphon effect is negligible in LP shunts."
The statement that the siphon effect is negligible if the inflow site (lumbar) and the outflow site (peritoneal) are at the same level in the standing position is formally correct, but seems misleading. The impression is created here that the hydrostatic pressure conditions in the standing position are irrelevant for the LP shunt and that no countermeasure (as in the VP shunt) is required. In the case of the LP shunt, although there is indeed no longer a "hanging column of fluid" BELOW the valve, the entire weight of the fluid column is present ABOVE the valve. This statement may be confusing, since there is no fluid-filled catheter at the top (as in the VP shunt). However, the same hydrostatic pressure exists in the (fluid-filled) spinal canal, which increases downward in proportion to the distance from the ventricles ("p =r *g*h"). However, for the valve, which is always a differential pressure valve, only the difference between the pressures at the inlet and outlet plays the decisive role. Thus, it does not matter whether the valve is opened while standing by a strong additional suction at the outlet ("siphoning") or by an increased pressure at the inlet. Therefore, the following formulation by Mirone et al. is more appropriate (29):
"Lumboperitoneal shunts may produce similar posture-related problems. The difference is that the hydrostatic pressure does not produce pulling but rather pushing force in the vertical body position."
The fact remains that with both the VP and LP shunt, an additional, strong differential pressure must be taken into account, which only occurs in the upright position. This unavoidably opens the valve and can thus lead to overdrainage. The hydrostatic pressure conditions are shown in Figure 5.
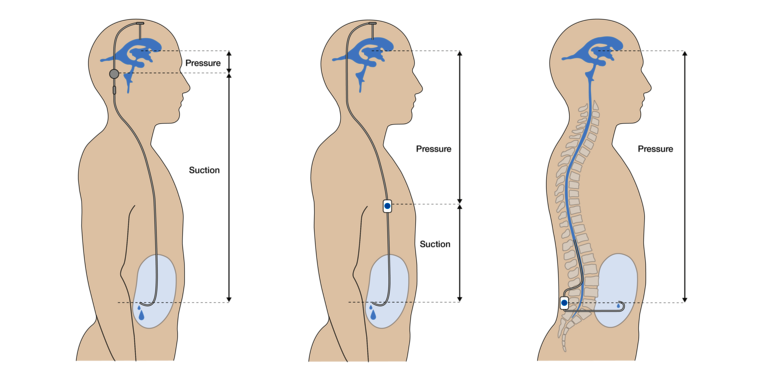
Various authors have shown that the implantation of an anti-siphon device (ASD) is therefore also necessary and effective in the LP shunt. Various gravitational valves and the so-called SiphonGuard have been used for this purpose (23,28,30). In a standing position, the SiphonGuard does not completely prevent (over)drainage, but it reduces the flow and thus slows down its development. In addition, the use of flow restrictors in the form of narrow lumen catheters (inner diameter 0.8 mm) to prevent overdrainage has also been described. These generally reduce flow in the LP shunt, i.e., regardless of body position. However, a recent comparative study by Nakajima with 115 included iNPH patients suggests a superiority of gravitational valves (30). The membrane-based ASDs mentioned by Miyake (Delta® Chamber & ASD) represent a special case, as they respond differently to pressure at the inlet or suction at the outlet. Their function depends on the appropriate inlet pressure/outlet suction ratio, and thus essentially on the appropriate implantation height in the body. Therefore, such membrane valves can indeed to be considered problematic for LP shunts (26).
Figure 6 shows a schematic comparison of the dynamic relationships within the VP and LP shunts. In the VP shunt, CSF is drained directly from the ventricles into the abdomen. If slit ventricles form due to overdrainage, the ventricular catheter, which is then trapped by tissue, is particularly vulnerable to occlusion. Due to the different type of drainage, this is not possible with the LP shunt. Here, the excess CSF first flows through the IV. ventricle into the subarachnoid and then into the spinal space before being drained from there into the abdominal cavity. Obstruction of the lumbar catheter by tissue entrapment is less likely here. However, the flow movement from the upper three ventricles through the IV. ventricle into the spinal canal carries the risk of entrainment/displacement of certain brain parts, which can lead to equally dangerous herniation (see flow direction LP shunt Fig. 6). This relationship becomes very clear in non-communicating hydrocephalus, especially due to an occluded aqueduct. The overpressure prevailing in the upper three ventricles would move the whole brain towards the spinal canal as soon as the spinal canal would be opened. For this reason, non-communicating hydrocephalus is also an important contraindication for LP shunting (13).
Valve types for the LP Shunt
As described, the differential pressure valves that can be used for an LP shunt do not differ in their technical function from those for a VP shunt. No special technical requirements for LP valves are described in the literature and, in principle, any differential pressure valve can be used for this purpose.
However, the implantation site in the particularly dynamic lumbar region of the body results in some special requirements for the mechanical stability of an LP valve, especially for its subcutaneous fixation. The valve of a VP shunt, implanted under thin immobile scalp on solid skull bone, is subjected to much lower mechanical stress compared to the valve of an LP shunt. In the case of the LP shunt, it is essential to prevent possible migration of the valve and thus catheter breakage by fixation in the tissue. The mechanical resistance of the valve body to fracture caused by possible bending is also important here. For gravitational valves, a change in the angle of inclination (twisting) must also be ruled out, as they must be aligned exactly with the body axis. Therefore, fixation eyelets on the valve that can be sutured to the subcutaneous tissue are advantageous. Mirone et al. mention here, for example, the Integra™ Horizontal-Vertical (H-V) Lumbar Valve and the MIETHKE DUAL SWITCH Valve (DSV) as suitable valves (29). For adjustable valves, which are predominantly used nowadays, too great an implantation depth should also be avoided so as not to impair the magnetic adjustment function. In addition, a firm support or abutment should be provided in case an adjustment becomes necessary, which can be achieved, for example, by implantation on the fascia or the costal arch. Likewise, valves with an as wide as possible base area or an implantable wide-area valve mounting device can be used for this purpose. This largely counteracts the valves "sinking" into the housing during the adjustment process. Miyake used these criteria to assess the suitability of some widely used adjustable valves as LP shunts. The result is shown in Figure 7 (26).
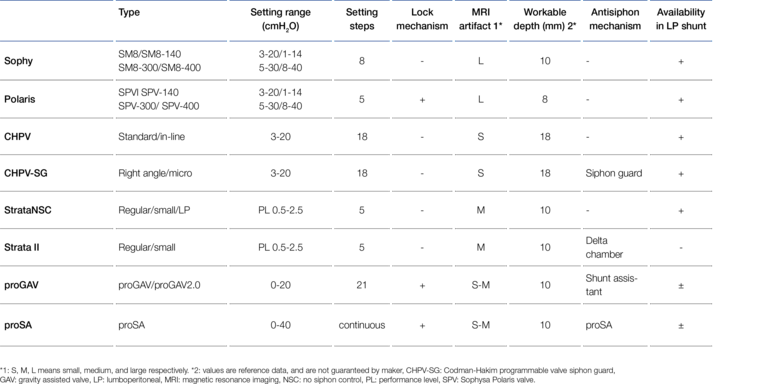
According to Miyake's assessment in 2016, the MIETHKE proGAV® and the proSA® valve could only be used in LP shunts to a limited extent because they could not be fixed directly subcutaneously. Since 2023, however, this has been possible with the so-called " valve board", an implantable abutment made of biocompatible silicone.

Literature
1. Crea A, Bianco A, Cossandi C, Forgnone S, Fornaro R, Crobeddu E, Marino D, Piras G, Scalia G, Saglietti C, Boldorini R, Galzio R, Panzarasa G. Choroid Plexus Carcinoma in Adults: Literature Review and First Report of a Location into the Third Ventricle. World Neurosurgery. 2020 Jan; 133:302-307. doi: 10.1016/j.wneu.2019.10.051.
2. Desai B, Hsu Y, Schneller B, Hobbs JG, Mehta AI, Linninger A. Hydrocephalus: the role of cerebral aquaporin-4 channels and computational modeling considerations of cerebrospinal fluid. Neurosurg Focus. 2016 Sep; 41(3): E8. doi: 10.3171/2016.7.FOCUS16191
3. Mazzola CA, Choudhri AF, Auguste KI, Limbrick DD, Jr., Rogido M, Mitchell L, Flannery AM, Pediatric Hydrocephalus Systematic R. and Evidence-Based Guidelines Task F. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: Management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr. 2014 Nov; 14(1): 8-23. doi: 10.3171/2014.7.PEDS14322
4. Zhu X, Di Rocco C. Choroid plexus coagulation for hydrocephalus not due to CSF overproduction: a review. Childs Nerv Syst. 2013 Jan; 29(1): 35-42. doi: 10.1007/s00381-012-1960-0
5. Cartwright CC and Wallace DC. Nursing care of the pediatric neurosurgery patient. Springer-Verlag; 2007. Available from: doi: 10.1007/978-3-642-32554-0
6. Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Review. 1999 Oct; 22(2-3): 67-93; discussion 94-65. doi: 10.1007/s101430050035
7. Giordan E, Palandri G, Lanzino G, Murad MH, Elder BD. Outcomes and complications of different surgical treatments for idiopathic normal pressure hydrocephalus: a systematic review and meta-analysis. Journal of Neurosurgery. 2018 Nov 1; 1-13. doi: 10.3171/2018.5.JNS1875
8. Hayhurst C, Beems T, Jenkinson MD, Byrne P, Clark S, Kandasamy J, Goodden J, Nandoe Tewarie RD, Mallucci CL. Effect of electromagnetic-navigated shunt placement on failure rates: a prospective multicenter study. Journal of Neurosurgery. 2010 Dec; 113(6): 1273-1278. doi: 10.3171/2010.3.JNS091237
9. Janson CG, Romanova LG, Rudser KD and Haines SJ. Improvement in clinical outcomes following optimal targeting of brain ventricular catheters with intraoperative imaging: Clinical article. Journal of Neurosurgery. 2014 Mar; 120(3): 684-696. doi: 10.3171/2013.8.JNS13250
10. Tuli S, O'Hayon B, Drake J, Clarke M, Kestle J. Change in ventricular size and effect of ventricular catheter placement in pediatric patients with shunted hydrocephalus. Neurosurgery. 1999 Dec; 45(6): 1329-1333; discussion 1333-1325. doi: 10.1097/00006123-199912000-00012
11. Abubaker K, Ali Z, Raza K, Bolger C, Rawluk D, O'Brien D. Idiopathic intracranial hypertension: lumboperitoneal shunts versus ventriculoperitoneal shunts--case series and literature review. British Journal of Neurosurgery. 2011 Feb; 25(1): 94-99. doi: 10.3109/02688697.2010.544781
12. Azad TD, Zhang Y, Varshneya K, Veeravagu A, Ratliff JK, Li G. Lumboperitoneal and Ventriculoperitoneal Shunting for Idiopathic Intracranial Hypertension Demonstrate Comparable Failure and Complication Rates. Neurosurgery. 2020 Feb; 86(2): 272-280. doi: 10.1093/neuros/nyz080
13. Yadav Y. R., Parihar V, Sinha M. Lumbar peritoneal shunt. Neurol India. 2010;58(2): 179-184. DOI: 10.4103/0028-3886.63778
14. Marupudi NI, Harris C, Pavri T, Mell B, Singh R, Ham SD and Sood S. The role of Lumboperitoneal shunts in managing chronic hydrocephalus with slit ventricles. Journal of Neurosurgery Pediatrics. 2018 Dec 1; 22(6): 632-637. doi: 10.3171/2018.6.PEDS17642
15. Cheng CH, Lin HL, Chuang HY. Tonsillar herniation as a complication of Lumboperitoneal shunt: case report and literature review. British Journal of Neurosurgery. 2018 Dec 6; 1-4. doi: 10.1080/02688697.2018.1538481
16. Beeravolu L, Ahmed I. Complications from management of idiopathic intracranial hypertension: A case of CSF overdrainage from a Lumboperitoneal shunt. Cephalalgia. 2016; 36: 108
17 . Miyajima M, Kazui H, Mori E, Ishikawa M, Sinphoni-Investigators o. b. o. t. One-year outcome in patients with idiopathic normal-pressure hydrocephalus: comparison of Lumboperitoneal shunt to ventriculoperitoneal shunt. Journal of Neurosurgery. 2016 Dec; 125(6): 1483-1492. doi: 10.3171/2015.10.JNS151894
18. Kandasamy J, Hayhurst C, Clark S, Jenkinson MD, Byrne P, Karabatsou K, Mallucci CL. Electromagnetic stereotactic ventriculoperitoneal csf shunting for idiopathic intracranial hypertension: a successful step forward? World Neurosurgery. 2011 Jan 7; 75(1): 155-160; discussion 132-153. doi: 10.1016/j.wneu.2010.10.025
19. Karabatsou K, Quigley G, Buxton N, Foy P, Mallucci C. Lumboperitoneal shunts: are the complications acceptable? Acta neurochirurgica (Wien). 2004 Nov; 146(11): 1193-1197. doi: 10.1007/s00701-004-0392-3
20. Menger RP, Connor DE Jr., Thakur JD, Sonig A, Smith E, Guthikonda B, Nanda A. A comparison of Lumboperitoneal and ventriculoperitoneal shunting for idiopathic intracranial hypertension: an analysis of economic impact and complications using the Nationwide Inpatient Sample. Neurosurgical Focus. 2014 Nov; 37(5): E4. doi: 10.3171/2014.8.FOCUS14436
21. Kazui H, Miyajima M, Mori E, Ishikawa M and Investigators S. Lumboperitoneal shunt surgery for idiopathic normal pressure hydrocephalus (SINPHONI-2): an open-label randomised trial. The Lancet Neurology. 2015 Jun; 14(6): 585-594. doi: 10.1016/S1474-4422(15)00046-0
22. Ho YJ, Chiang WC, Huang HY, Lin SZ and Tsai ST. Effectiveness and safety of ventriculoperitoneal shunt versus lumboperitoneal shunt for communicating hydrocephalus: A systematic review and meta-analysis with trial sequential analysis. CNS neuroscience & therapeutics. 2023 Jan 17; 29(3), 804–815. doi: 10.1111/cns.14086
23. Bloch O, McDermott MW. Lumboperitoneal shunts for the treatment of normal pressure hydrocephalus. Journal of the Neurosurgical Society of Australasia. 2012 Aug; 19(8): 1107-1111. doi: 10.1016/j.jocn.2011.11.019
24. Nakajima M, Miyajima M, Ogino I, Akiba C, Kawamura K, Kurosawa M, Kuriyama N, Watanabe Y, Fukushima W, Mori E, Kato T, Sugano H, Karagiozov K and Arai H. Shunt intervention for possible idiopathic normal pressure hydrocephalus improves patient outcomes: A nationwide hospital-based survey in Japan. Frontiers in Neurology. 2018 Jun; 9(421). doi: 10.3389/fneur.2018.00421
25. Jusue-Torres I, Hoffberger J, Rigamonti D. Chapter 14: Complications specific to Lumboperitoneal Shunt, in Complications of CSF Shunting in Hydrocephalus: Prevention, Identification and Management, p. edited by C. Di Rocco, M. Turgut, G. Jallo and J. F. Martinez Lage, Publisher: Springer, Heidelberg, London, New York, 2015,
26. Miyake H. Shunt Devices for the Treatment of Adult Hydrocephalus: Recent Progress and Characteristics. Neurologia medico-chirurgica (Tokyo). 2016 May 15; 56(5): 274-283. doi: 10.2176/nmc.ra.2015-0282
27. Marupudi NI, Pavri T, Harris C, Haridas A, Ham S, Sood S. Lumbar-Peritoneal Shunting in Hydrocephalus: Preventing Chiari Malformations and Decreasing Shunt Revisions. Journal of Neurosurgery. 2014 Apr; 124(4)
28. Kanazawa R, Ishihara S, Sato S, Teramoto A, Kuniyoshi N. Familiarization with lumboperitoneal shunt using some technical resources. World Neurosurgery. 2011 Sep-Oct; 76(3-4): 347-351. doi: 10.1016/j.wneu.2011.02.024
29. Mirone G, Spina D, Sainte-Rose C. Shunt hardware, in Pediatric Hydrocephalus. p. edited by G. Cinalli, Springer; 2019.
30. Nakajima M, Miyajima M, Akiba C, Ogino I, Kawamura K, Sugano H, Hara T, Tange Y, Fusegi K, Karagiozov K, Arai H. Lumboperitoneal Shunts for the Treatment of Idiopathic Normal Pressure Hydrocephalus: A Comparison of Small-Lumen Abdominal Catheters to Gravitational Add-On Valves in a Single Center. Operative neurosurgery (Hagerstown, Md.). 2018 Dec 1; 15(6): 634-642. doi: 10.1093/ons/opy044